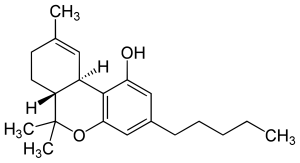
The U.S Drug Enforcement Administration has relaxed its regulations on medical marijuana research. The new regulations allow researches to skip a step in the approval process if they want to modify their studies by obtaining more CBD- or cannabinol – an active chemical in marijuana.
This change essentially means less paperwork is needed and the process is sped up to obtain more CBD for research purposes.
The Pennsylvania Medical Society believes this change will open many doors for future research opportunities. They would prefer the ability to conduct more research and clinical studies before the state makes any moves to permit medical use.
Many say this is a small step in the right direction.
The DEA’s new rule only applies to CBD, not THC – trahydrocannabinol – the common component of marijuana that causes psychological effects. This shows that agency officials are still being resistant when making decisions related to marijuana research.
While a step in the right direction, there are still many rules prohibiting the study of both THC and CBD, where some patients may in fact need both.
We can expect to continue to see changes with regulations in the marijuana industry, and hopefully this new availability for researchers can lead to future answers on the drug.






Recent Comments